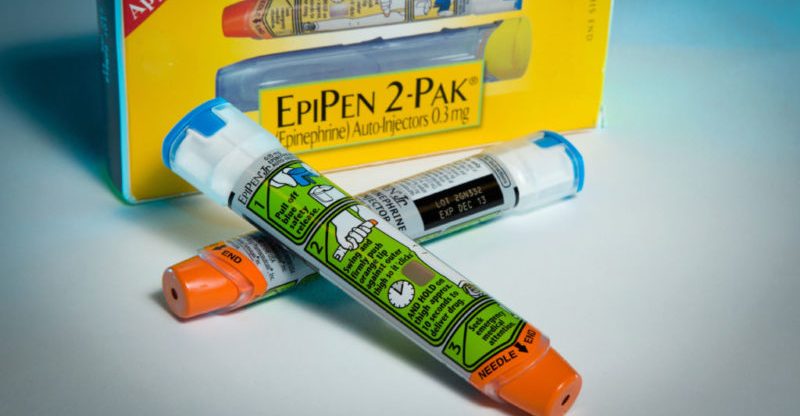
Mylan Finalizes $465 Million EpiPen Settlement With Justice Department.
Mylan NV (MYL.O) has finished a $465 million settlement settling U.S. Justice Department claims it cheated the administration for its EpiPen emergency allergy treatment, which turned into the focal point of a firestorm over cost increments.
The U.S. Lawyer’s Office in Massachusetts on Thursday reported the agreement, which was not long after scrutinized by some congressional individuals as being too simple on the drugmaker. It came 10 months after Mylan said it had achieved a deal.
The settlement settled cases that Mylan avoided higher rebates to state Medicaid programs by misclassifying EpiPen as a generic product, despite the fact that it was priced and marketed as a brand-name product.
Mylan admitted wrongdoing in going into the settlement. It will rename EpiPen and pay the rebate appropriate to its new characterization as of April 1, 2017.
The arrangement took after a False Claims Act whistleblower lawsuit filed by French adversary Sanofi SA (SASY.PA) in 2016, two years after it initially raised the issue with authorities, Weinreb’s office said.
Sanofi, which once advertised an opponent product called Auvi-Q, will get about $38.8 million as a reward from the government.
The EpiPen, which Mylan procured in 2007, is a handheld gadget that treats life-threatening allergic reactions via automatically injecting a dosage of epinephrine.
Mylan experienced harsh criticism a year ago subsequent to raising the cost of a couple of EpiPens to $600, from $100 in 2008, enraging customers and placing it in the center of the continuous debate over the high cost of prescribed medicines in the United States.
Mylan has since offered its own generic version for about $300.
The Justice Department settlement fixated on claims that Mylan kept away from higher refunds to state Medicaid programs by misclassifying the EpiPen as a generic item, even though the company marketed and valued it as a brand-name item.
A U.S. Department of Health and Human Services’ Office of Inspector General examination discharged in May found the U.S. government may have overpaid for EpiPens by up to $1.27 billion in the vicinity of 2006 and 2016.